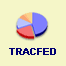
All Agencies, Latest Month All Agencies, Current FY Geographic Distribution
of Convictions for
All Agencies, FY 2024
87 Prosecutions
in September 2024
1,048 Prosecution
in Fiscal Year 2024
83 Convictions
in September 2024
924 Convictions
in Fiscal Year 2024CITE
21 USC Sec. 952 01/05/2009EXPCITE
TITLE 21 - FOOD AND DRUGS CHAPTER 13 - DRUG ABUSE PREVENTION AND CONTROL SUBCHAPTER II - IMPORT AND EXPORTHEAD
Sec. 952. Importation of controlled substancesSTATUTE
(a) Controlled substances in schedule I or II and narcotic drugs in schedule III, IV, or V; exceptions It shall be unlawful to import into the customs territory of the United States from any place outside thereof (but within the United States), or to import into the United States from any place outside thereof, any controlled substance in schedule I or II of subchapter I of this chapter, or any narcotic drug in schedule III, IV, or V of subchapter I of this chapter, or ephedrine, pseudoephedrine, or phenylpropanolamine, except that - (1) such amounts of crude opium, poppy straw, concentrate of poppy straw, and coca leaves, and of ephedrine, pseudoephedrine, and phenylpropanolamine, as the Attorney General finds to be necessary to provide for medical, scientific, or other legitimate purposes, and (2) such amounts of any controlled substance in schedule I or II or any narcotic drug in schedule III, IV, or V that the Attorney General finds to be necessary to provide for the medical, scientific, or other legitimate needs of the United States - (A) during an emergency in which domestic supplies of such substance or drug are found by the Attorney General to be inadequate, (B) in any case in which the Attorney General finds that competition among domestic manufacturers of the controlled substance is inadequate and will not be rendered adequate by the registration of additional manufacturers under section 823 of this title, or (C) in any case in which the Attorney General finds that such controlled substance is in limited quantities exclusively for scientific, analytical, or research uses, may be so imported under such regulations as the Attorney General shall prescribe. No crude opium may be so imported for the purpose of manufacturing heroin or smoking opium. (b) Nonnarcotic controlled substances in schedule III, IV, or V It shall be unlawful to import into the customs territory of the United States from any place outside thereof (but within the United States), or to import into the United States from any place outside thereof, any nonnarcotic controlled substance in schedule III, IV, or V, unless such nonnarcotic controlled substance - (1) is imported for medical, scientific, or other legitimate uses, and (2) is imported pursuant to such notification, or declaration, or in the case of any nonnarcotic controlled substance in schedule III, such import permit, notification, or declaration, as the Attorney General may by regulation prescribe, except that if a nonnarcotic controlled substance in schedule IV or V is also listed in schedule I or II of the Convention on Psychotropic Substances it shall be imported pursuant to such import permit requirements, prescribed by regulation of the Attorney General, as are required by the Convention. (c) Coca leaves In addition to the amount of coca leaves authorized to be imported into the United States under subsection (a) of this section, the Attorney General may permit the importation of additional amounts of coca leaves. All cocaine and ecgonine (and all salts, derivatives, and preparations from which cocaine or ecgonine may be synthesized or made) contained in such additional amounts of coca leaves imported under this subsection shall be destroyed under the supervision of an authorized representative of the Attorney General. (d) Application for increased importation of ephedrine, pseudoephedrine, or phenylpropanolamine (1) With respect to a registrant under section 958 of this title who is authorized under subsection (a)(1) to import ephedrine, pseudoephedrine, or phenylpropanolamine, at any time during the year the registrant may apply for an increase in the amount of such chemical that the registrant is authorized to import, and the Attorney General may approve the application if the Attorney General determines that the approval is necessary to provide for medical, scientific, or other legitimate purposes regarding the chemical. (2) With respect to the application under paragraph (1): (A) Not later than 60 days after receiving the application, the Attorney General shall approve or deny the application. (B) In approving the application, the Attorney General shall specify the period of time for which the approval is in effect, or shall provide that the approval is effective until the registrant involved is notified in writing by the Attorney General that the approval is terminated. (C) If the Attorney General does not approve or deny the application before the expiration of the 60-day period under subparagraph (A), the application is deemed to be approved, and such approval remains in effect until the Attorney General notifies the registrant in writing that the approval is terminated. (e) Reference to ephedrine, pseudoephedrine, or phenylpropanolamine Each reference in this section to ephedrine, pseudoephedrine, or phenylpropanolamine includes each of the salts, optical isomers, and salts of optical isomers of such chemical.SOURCE
(Pub. L. 91-513, title III, Sec. 1002, Oct. 27, 1970, 84 Stat. 1285; Pub. L. 95-633, title I, Sec. 105, Nov. 10, 1978, 92 Stat. 3772; Pub. L. 98-473, title II, Secs. 519-521, Oct. 12, 1984, 98 Stat. 2075; Pub. L. 109-177, title VII, Sec. 715, Mar. 9, 2006, 120 Stat. 264.)REFERENCES IN TEXT
Schedules I, II, III, IV, and V, referred to in subsecs. (a) and (b), are set out in section 812(c) of this title.AMENDMENTS
2006 - Subsec. (a). Pub. L. 109-177, Sec. 715(1)(A), inserted "or ephedrine, pseudoephedrine, or phenylpropanolamine," after "schedule III, IV, or V of subchapter I of this chapter," in introductory provisions. Subsec. (a)(1). Pub. L. 109-177, Sec. 715(1)(B), inserted ", and of ephedrine, pseudoephedrine, and phenylpropanolamine," after "coca leaves". Subsecs. (d), (e). Pub. L. 109-177, Sec. 715(2), added subsecs. (d) and (e). 1984 - Subsec. (a)(1). Pub. L. 98-473, Sec. 519, amended par. (1) generally, inserting references to poppy straw and concentrate of poppy straw. Subsec. (a)(2)(C). Pub. L. 98-473, Sec. 520, added subpar. (C). Subsec. (b)(2). Pub. L. 98-473, Sec. 521, substituted "is imported pursuant to such notification, or declaration, or in the case of any nonnarcotic controlled substance in schedule III, such import permit, notification, or declaration, as the Attorney General may by regulation prescribe, except that if a nonnarcotic controlled substance in schedule IV or V is also listed in schedule I or II of the Convention on Psychotropic Substances it shall be imported pursuant to such import permit requirements, prescribed by regulation of the Attorney General, as are required by the Convention" for "is imported pursuant to such notification or declaration requirements as the Attorney General may by regulation prescribe, except that if a nonnarcotic controlled substance in schedule III, IV, or V is also listed in schedule I or II of the Convention on Psychotropic Substances it shall be imported pursuant to such import permit requirements, prescribed by regulation of the Attorney General, as are required by the Convention". 1978 - Subsec. (b)(2). Pub. L. 95-633 inserted provision relating to exception for nonnarcotic controlled substances listed in schedule I or II of the Convention on Psychotropic Substances. EFFECTIVE DATE OF 1978 AMENDMENT Amendment by Pub. L. 95-633 effective on date the Convention on Psychotropic Substances enters into force in the United States [July 15, 1980], see section 112 of Pub. L. 95-633, set out as an Effective Date note under section 801a of this title.
 |
 |
 |
 |
 |
 |
 |
 |
Copyright 2010